As anyone who has enjoyed a fizzy drink knows, CO2 dissolves in water – refreshing news for our gullets, and good news for our planet. With more than 70% of the Earth’s surface covered by the water in our ocean, this basic physical process acts as a brake on global warming on a massive scale.
Since the dawn of the Industrial Revolution, a quarter of humanity’s CO2 emissions from the burning of fossil fuels have been absorbed by the ocean. The seas, in other words, have helped to clean up our mess. In doing so, they have lowered greenhouse gas levels in the atmosphere and limited global warming.
Unfortunately, as the rate of human-driven emissions has increased, the oceans have been unable to store carbon away quickly enough to prevent warming entirely. But could they?
Some scientists believe the carbon-uptake of the oceans can be artificially accelerated. They have suggested a range of technologies, from the complex to the very simple. If successful, they could help us in coming decades as the world struggles to move towards a low-carbon economy.
Ocean-based carbon dioxide removal (CDR) schemes, as they are called, are controversial. They are mostly untested – certainly at the scale that would be required – and could come with damaging side effects. They could be expensive and might act as a distraction from the urgent need to reduce emissions. But they could also offer a helping hand when we most need it.
As the profile of such CDR proposals rises, a growing number of scientists are weighing up their risks and benefits. A key report from the US National Academies of Sciences, Engineering and Medicine has identified the following possible methods as the most promising.
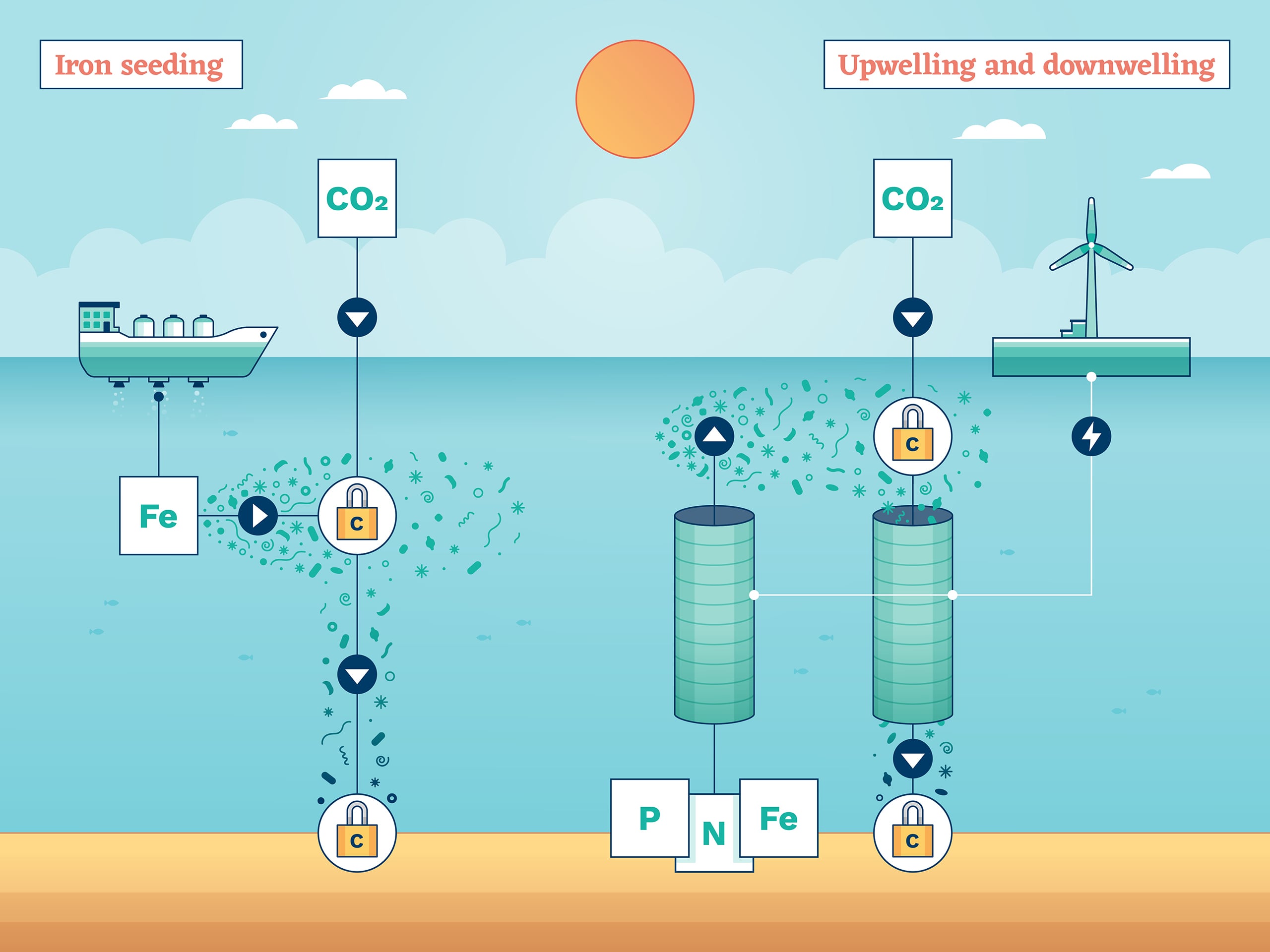
Iron seeding
One of the oldest CDR ideas, iron seeding, also known as iron fertilisation, seeks to exploit the natural use of carbon by huge swarms of photosynthetic plankton.
Just like trees on land, these plankton use sunlight and CO2 to produce energy and grow. In doing so, they incorporate the carbon into their microscopic bodies. And when they die and sink to the deep ocean, they take it with them.
That doesn’t sound like much. But given there are an estimated octillion – a billion billion billion – phytoplankton spread across the global ocean, they are the single biggest way that carbon from the atmosphere ends up in the oceans.
Phytoplankton need nutrients to grow, which can be in short supply in seawater. In fact, scientists know that the growth of many types of phytoplankton is limited by a lack of iron. The theory goes, if you want more phytoplankton and more carbon removal, just add iron to the top layer of the sea where the phytoplankton grow. Think of it as fertiliser. In some ways, this CDR method would imitate nature. Natural fertilisation with iron happens when currents bring nutrient-rich water up from the depths, or winds spread iron-containing dust and volcanic ash across the sea. One proposal to enhance this process is to boost the atmospheric content of iron with aerosols, though most trials dump iron sulphate from ships.
There are concerns over the environmental impacts of the addition of vast amounts of iron to the ocean, which could disturb the ocean ecosystem and have knock-on effects on food chains due to the growth of algal blooms. Some small-scale experiments have been carried out to test the idea and more are planned.
Upwelling and downwelling
Phytoplankton that live near the ocean surface remove carbon from the atmosphere and carry it to the deep sea when they die – a process helped when deep ocean currents bring up nutrient-rich water from below. So, instead of adding iron from above, maybe science and technology could give this natural fertilisation of phytoplankton a boost?
Artificial upwelling is a carbon removal technology that could achieve this. It’s another simple concept: vertical pipes in the ocean could bring deep water to the surface, where it could promote the extra growth of plankton. To help that upward flow, and to speed the deposit of carbon-rich water from the surface into the deep ocean, another set of pipes could carry water in the other direction. That’s called, quite predictably, artificial downwelling.
Less predictable is just how all this water could be moved around. The vertical pipes would need to be hundreds of metres long and up to 20 metres wide to shift enough water to make it worthwhile. Some plans have them moored to the seabed, and in others they float.
Pumping liquids is, however, expensive and energy intensive. So where would the energy come from? Might solar panels do the job? Could the action of waves at the surface help nudge the surface water down? Might the equipment break down or disintegrate and only add to the problem of ocean pollution? Many questions remain for a scheme that clearly has its ups and downs. Numerous small outdoor experiments in the deep ocean, lakes and fjords have taken place, on varying scales. There are concerns that, if performed on a large scale, the technique will alter the density and temperature profile of the ocean, with possible impacts on sea life.
Farming seaweed
Just like phytoplankton, seaweed takes CO2 and uses it to grow. In fact, phytoplankton are themselves a form of seaweed – a catch-all term used to describe a wide range of marine plants and algae.
Most plans to cultivate and farm seaweed focus on the plants that grow in rocky coastal waters, which presents a problem for carbon storage. Unlike the phytoplankton that can fall for thousands of metres through the deep sea and bury their carbon in seabed sediment, larger coastal seaweeds have long been thought to mostly disintegrate in shallow water and come to rest on hard stone surfaces at the bottom. These surfaces are more likely to be disturbed, making it more likely that this carbon will be re-released into the atmosphere. But some research says it’s more complicated than that, and that much carbon from coastal seaweed can indeed end up in deep-sea sediment.
Either way, just like planting trees on land, growing more seaweed is an attractive way to trap and store carbon for a while – the key is what to do with it next. Perhaps it could be harvested and used as biomass for energy. Or it could be fed to animals, or people. Incentives would be important: some proponents of seaweed cultivation for carbon storage argue that it should generate credits that can be sold to consumers and companies to offset their own greenhouse gas emissions. Seaweed farming is already done around the world, but it would need to be significantly scaled up to have an impact on climate. And some are anxious that setting up yet more infrastructure in the ocean could impact existing marine life.
Protecting and restoring ocean ecosystems
Mud, glorious mud. Unloved and unnoticed for years, sediment-rich coastal ecosystems that fringe the oceans are now a hot topic in carbon accounting. Mostly mangrove swamps, salt marshes and seagrass meadows, these mushy frontiers between the land and sea sit on a rich abundance of carbon. Organic matter in the form of leaves, wood, roots and dead marine life hold giant stocks of carbon that have built up over thousands of years.
A frequent casualty of coastal development, such ecosystems are often dug up, destroyed and drained, which can release all the carbon they had previously stored into the atmosphere. So, preventing further destruction by raising awareness of their value and protecting what’s left is an excellent start.
On top of that, it is possible to add to their carbon-carrying capacity, by restoring and replacing lost and degraded coastal ecosystems.
Two recent changes are helping achieve both protection and restoration. Firstly, these gloopy sediment-filled ecosystems have been rebranded, as the storers of so-called “blue carbon”, which sounds far more attractive. And secondly, blue carbon schemes are now eligible to generate carbon credits, which means companies are racing to hand over money to projects in exchange for offsets against their own greenhouse gas emissions.
But, much like their tree-based counterparts, the carbon removal realised through the restoration of blue carbon ecosystems is only long-lasting if they remain protected and undisturbed.
Boosting ocean alkalinity
Some chemistry: when seawater that contains dissolved CO2 becomes more alkaline – that is, less acidic – the change triggers a series of chemical reactions. The end result is that the CO2 converts to different molecules, including carbonates and bicarbonates.
This is good for two reasons. First, these new molecules are more stable and so more likely to keep their carbon tied up and not release it back to the atmosphere. Second, removing dissolved CO2 enables the water to absorb more of the gas from the atmosphere, to take its place. Both these processes can help tackle global warming.
Also called weathering, this process happens naturally as alkaline rocks or shells from sea creatures slowly dissolve in seawater. So, perhaps we can speed it up? The plan would be simple: add crushed alkaline minerals and materials, such as lime, to beaches to be weathered and washed into the sea, or directly into the ocean from ships. There would be another environmental benefit too: the added alkaline material would combat the rising acidification that is resulting from the ocean absorbing humanity’s carbon emissions. On the other hand, mining for the necessary minerals would be a destructive practice, and deliberately adding them to the sea could see a build-up of trace toxins in sea life. Still, field trials are planned.
Seawater electrification
More chemistry: passing an electric current through seawater causes molecular chaos. The charge breaks the bond between the hydrogen and oxygen molecules of water (H2O, of course), and when liberated, these separate components trigger reactions, including with the sodium and chlorine of salt (NaCl).
These reactions can be harnessed to free CO2 from seawater in a few ways. Commonly discussed are: a method that captures a stream of CO2 gas liberated from seawater, which could be collected and stored; and a way to generate alkalinity, which encourages the chemical conversion of dissolved CO2 to more stable carbonate forms.
Both these ideas leave behind seawater that contains a lower content of dissolved CO2 than before, leaving it primed to absorb more from the atmosphere. So, a system could work something like this: pump in seawater to a treatment facility, electrify it, pump it back to the ocean. Whether that could be done in a cheap and sustainable way depends on the details – not least finding a renewable way to generate the required electricity.
Modelling and theoretical calculations suggest the idea could work, and some researchers now want to build the equipment needed to demonstrate the technique.